valence electrons of barium|Iba pa : Clark Mar 23, 2023 Mary rose pinay sariling sikap habang naliligo 40 sec. 40 sec Jeet0919 - 360p. boso kay hipag 53 sec. 53 sec Judez415 - 360p. Nagshave si hipag 63 sec. 63 sec Judez415 - 720p. Boso Naliligo sobrang puti 28 sec. 28 sec Tsupain Moko - 720p. Kinantot si misis habang naliligo 4 min. 4 min Bisayaxxx - 360p. Video ni gf habang naliligo 2 min.
valence electrons of barium,Mar 23, 2023 There are two ways to find the number of valence electrons in Barium (Ba). The first is to use the Periodic Table to figure out how many electrons Barium has in its valence shell. .valence electrons of barium Iba pa Valences of the Elements Chemistry Table. You may assume that the .The periodic table and Lewis diagrams. Determine valence electrons using the periodic table. Google Classroom. Learn how to determine the number of valence electrons for .The name comes from the Greek 'barys', meaning heavy. Allotropes. Ba. Barium. 56. 137.327. Glossary. GroupA vertical column in the periodic table. Members of a group .

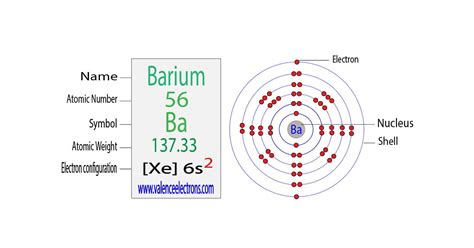
Barium has 2 valence electrons because there are 2 electrons present in the outermost shell of the Barium (Ba) atom. Now let’s see how you can easily find the .
Barium has 2 valence electrons. Methods. We can write the valence electrons of barium using two different methods: #1 Using periodic table. #2 Using electron configuration. Let’s break down each .Barium is soft, reactive, silvery-white alkaline-earth metal with two valence electrons. The element’s electronegativity is measured as a 0.9 (Pauling Scale) and it is more reactive than strontium and calcium. In nature, this . Electron configuration of Barium is [Xe] 6s2. Possible oxidation states are +2. Electron Configuration. The periodic table is a tabular display of the chemical .The electron configuration can be visualized as the core electrons, equivalent to the noble gas of the preceding period, and the valence electrons (e.g. [Xe] 6s2 for barium). Oxidation States Oxidation states are typically represented by integers which may be positive, zero, or negative.
Iba paThe element Barium was discovered by W. Scheele in year 1772 in United Kingdom. Barium was first isolated by H. Davy in 1808. How many valence electrons does a Barium atom have? Barium has 2 valence . Barium has 2 valence electrons and it loses these 2 electrons during a chemical reaction to become stable. When barium is kept open in air, it quickly reacts with oxygen and produces barium oxide. (Because of .
The diagram below shows the number of valence electrons (VE) for the main group elements. A periodic table showing how many valence electrons the main groups have. Group 1 = 1 valence electron Group 2 = 2 valence electrons Group 13 = 3 valence electrons Group 14 = 4 valence electrons Group 15 = 5 valence electrons Group 16 = .

Barium is soft, reactive, silvery-white alkaline-earth metal with two valence electrons. The element’s electronegativity is measured as a 0.9 (Pauling Scale) and it is more reactive than strontium and calcium. In nature, this element is always found combined with other elements. Barium is located below strontium and above radium on the .The atomic number of barium is 56, which means it has 56 electrons. Now it is possible to find the orbital notation of barium very easily through electron configuration. That is, the orbital notation of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2.
Explanation: Barium is a heavy alkaline earth metal, and is the fifth row congener of beryllium, magnesium, calcium, and strontium. It has surprisingly little industrial importance according to this link. Barium is an alkaline earth metal; i.e. Group II of the Periodic table. Therefore, it has 2 valence electrons.Contributors and Attributions. 3.10: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons .. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
valence electrons of barium Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in Figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable .
As a gas or vapor, the halogens all had a pungent odor. After the development of quantum mechanics, it was shown that the halogens all had seven valence electrons, supporting their original placement into the same group on Mendeleev's periodic table. Figure 11.1.1 11.1. 1: Periodic table by Dmitri Mendeleev, 1871.
Barium is a chemical element of the periodic table with chemical symbol Ba and atomic number 56 with an atomic weight of 137.328 u and is classed as a alkaline earth metal. . Valence electrons : 2: Valency electrons : 2: Bohr model: Electron shell for Barium, created by Injosoft AB Ba. Figure: Shell diagram of Barium (Ba) atom. Orbital .
Solution. Verified by Toppr. Barium is an alkaline earth metal; i.e. Group II of the Periodic table. Therefore, it has 2 valence electrons. Was this answer helpful? Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
Valence electrons are responsible for the reactivity of an element. They determine how "willing" the elements are to bond with each other to form new compounds. If the valence shell of an element is full, such as with a noble gas, then the element does not want to gain or lose an electron. For example, alkali metals, which all have a valency of .Create your account. View this answer. A barium (Ba) atom has 2 valence electrons. Valence electrons are the outermost electrons in an atom, which affect how atoms might react with one. See full answer below. The barium electron configuration, represented as 6s2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2, illustrates the arrangement of electrons within . Similarly, the ‘p’ in p block represents that all p block elements have their valence electrons in p subshell. And so on for d block and f block. Second, mark location of . The Lewis structure of Barium cation is reliable in showing the ability of the element for making ionic bonds with non-metals. Barium 2+ ion influence atomic structure to be described through Lewis Dot structure. The dots are the notation of symbol of the electrons that are lost by barium metal.
2. Find the electron configuration for the element you are examining. Once you know an element's electron configuration, finding its number of valence electrons is quite simple (except, of course, for the transition metals.) If you're given the configuration from the get-go, you can skip to the next step.
valence electrons of barium|Iba pa
PH0 · valence electrons chart
PH1 · valence electron configuration calculator
PH2 · number of valence electrons list
PH3 · list of valence electrons for each element
PH4 · how many valence electrons in titanium
PH5 · how do you find valence electrons
PH6 · Iba pa